The Translational Cancer Imaging (TCI) shared resource is an expansion of the previous Small Animal Imaging core. TCI continues to provide animal imaging support and now will facilitate human clinical research with state of the art imaging modalities and radioisotope production.

As new molecular targets are being developed for individualized therapy, similar “targeted imaging” will be needed to effectively assess treatment response, tumor heterogeneity, and mechanisms of resistance. The TCI will enable novel radiotracers to be developed along the entire translational path from development and synthesis to small animal studies, and to early clinical studies.
An exciting new aspect is that the TCI is equipped with full capabilities of producing and developing radionuclide imaging probes through the cyclotron housed in the Biomedical Research Imaging Center (BRIC), located in Marsico Hall. The cyclotron, a GE PETtrace 880, was installed in a heavily shielded vault in the subbasement of the Marsico Hall and certified in April, 2015 for routine radioisotope production. This cyclotron is capable of accelerating protons up to 16.5 MeV energies, which, after bombardment with specific targets, are used to produce commonly used PET radioisotopes including Fluorine-18, Carbon-11, Nitrogen-13, and Oxygen-15, as well as other radioisotopes. Right next to the cyclotron is the radiochemistry lab where the radioisotopes will be synthesized to various radioactive compounds for imaging. Automated delivery lines are in place to transfer radioisotopes directly from the cyclotron to shielded hot cells equipped with robot manipulators. Several synthesis modules (GE FastLab synthesizer) have been installed for automated production of radiocompounds, including 18F-FDG. Available radioactive tracers can be found in the BRIC webpage.
Run by new faculty Dr. Zibo Li, a radiochemist in the department of Radiology with a joint appointment in the BRIC, this facility is unique among Cancer Centers. “This brand new facility provides investigators the access to a broad spectrum of radiopharmaceuticals that could not only be used in preclinical research, but also in clinical research. Only a few centers have this capability in combination with the dedicated human PET/CT and PET/MRI research scanners”, said by Dr. Zibo Li. Dr. Li is available to provide consultation on making novel PET agents.
An important feature of the new facility is its full GMP compliance, which allows the production of imaging probes for human imaging in clinical research. The facility is outfitted well with quality control equipment, dedicated clean room and QC room, and Lab information management system (LIMS) for required reporting for human applications. Dr. Eric Smith, Department of Radiology and BRIC, is leading the radiopharmacy program and oversees regulatory requirements for human imaging studies. The new facility should greatly advance the translation from animal studies to human clinical research.
With this tremendous expansion, the TCI will provide more services and support on both preclinical and clinical research. We now offer as NEW services:
– various PET radiopharmaceuticals for clinic and preclinical studies, including 18F-FDG, 15O-H2O, 13N-NH3, 18F-FLT, 18F-FMISO, 11C-acetate, and 11C-choline
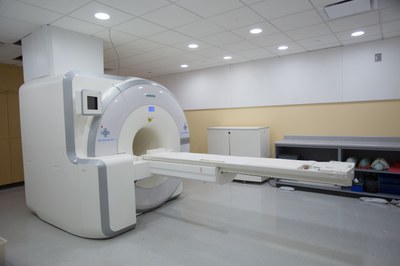
– MRI, PET/MRI, and PET/CT on human subjects for clinical research studies
– Regulatory assistance, including guidance of cGMP facility, QC, chemistry/ manufacturing/control documentation, and investigational new drug (IND) application of new PET agents
– Study consultation, IRB submission support for clinical research, and animal protocol submission support for animal studies, hands-on imaging training, technical support, and imaging education
– Develop new molecular probes based on investigator’s molecular target, including small molecule of peptides and aptamers, or large molecule of protein, antibodies and antibody fragment; providing probe design, radiolabeling optimization, and probe validation.
– Image quantification, including ROI measurements, time activity curve analysis, dynamic modeling, longitudinal analysis, and other methods to obtain meaningful parameters
This is in addition to many pre-clinical services still available:
– Provide optical, ultrasound, MRI, PET/CT, SPECT/CT, and high resolution CT imaging for preclinical imaging studies.
– Provide remote image access through PACS server, and image storage and archiving with data server.
– Image processing, including registration, segmentation, image classification, volume rendering, and other visualization needs.
Some key contacts for various needs of cancer imaging studies are listed below:
- Clinical imaging: Dr.Terry Wong, TCI Faculty Director, Department of Radiology and BRIC
- Radiochemistry and Imaging Probe Development: Dr. Zibo Li, TCI Faculty Co-Director, Department of Radiology and BRIC
- Radiopharmacy and Regulatory Issues: Dr. Eric Smith, Department of Radiology and BRIC
- Preclinical Imaging: Dr. Hong Yuan, Small Animal Imaging Facility Director, Department of Radiology and BRIC,
