UNC Lineberger’s Yuliya Pylayeva-Gupta, PhD, was the corresponding author of a paper published in Cell Reports Medicine that examined how B cells might be reprogrammed to improve anti-tumor immune response in pancreatic cancer. The study was led by a former research associate, Rahul Mirlekar.
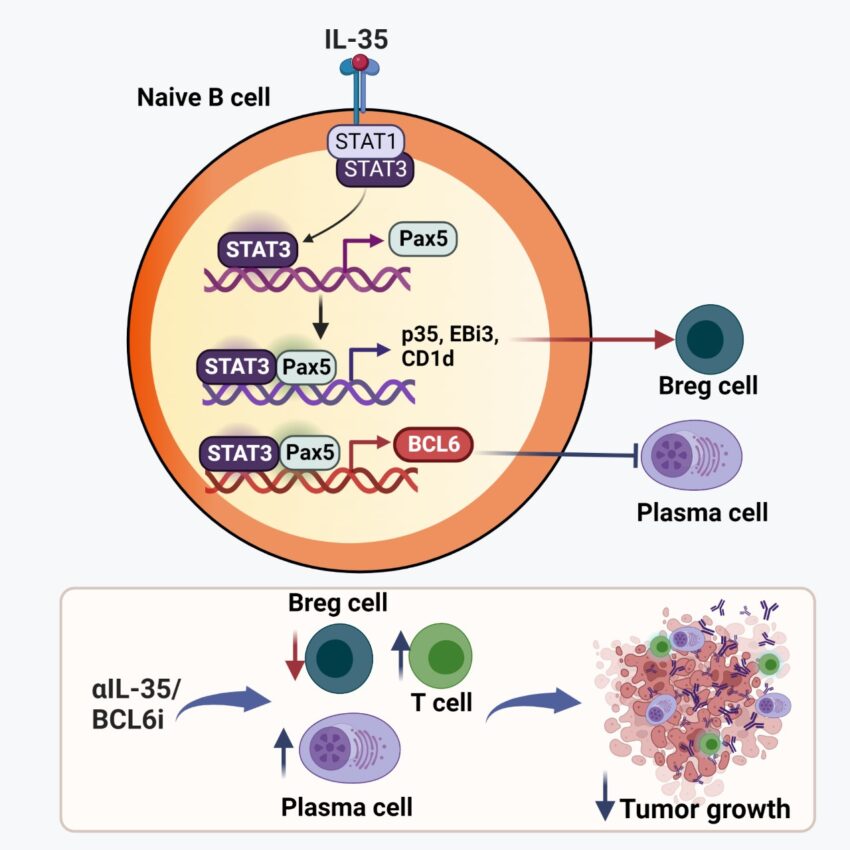
Pylayeva-Gupta and her co-authors including UNC Lineberger’s Naim Rashid, PhD, Jen Jen Yeh, MD, William Kim, MD, and Benjamin Vincent, MD, reported that IL-35-driven transcriptional reprogramming of naïve B cells in pancreatic cancer shifts their development away from effector plasma cell responses and toward an immunoregulatory phenotype. Inhibiting IL-35 or BCL6 in naïve B cells limits tumor growth by potentiating anti-tumor plasma and T cell responses.
Plasma cell response is associated with anti-tumor immunity and favorable response to immunotherapy. B cells can amplify anti-tumor immune responses through antibody production, but B cells in patients and tumor-bearing mice often fail to support these effector functions.
The researchers identified a dysregulated transcriptional program in B cells that disrupts differentiation of naïve B cells into anti-tumor plasma cells. Transient inhibition of BCL6 in tumor-educated naïve B cells is sufficient to reverse dysfunction in B cell differentiation, stimulating the intertumoral accumulation of plasma cells and effector T cells and rendering pancreatic tumors sensitive to anti-PD-1 blockade.
These findings suggest that B cell effector dysfunction in cancer might be caused by an active systemic suppression program that can be targeted to synergize with T cell-directed immunotherapy.
You can read the full article here.
—Tyler Rice, UNC Lineberger Pancreatic Cancer Center of Excellence