“In the places I go there are things that I see that I never could spell if I stopped with the Z. I’m telling you this ’cause you’re one of my friends. My alphabet starts where your alphabet ends!” (On Beyond Zebra, Dr. Seuss)
We encourage “critically creative” ideas on how to tackle one of the greatest problems in cancer…metastasis! Our inability to control metastatic spread is what leads to the death of nearly all cancer patients. Thus, our steadfast focus and interest is on understanding the complex processes involved in metastasis and to develop therapeutic strategies that attack its “Achilles’ heels”. 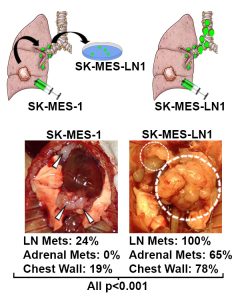
Role of Lymphatics in Metastasis:
The lymph node has long been known to carry prognostic relevance in cancer, and it part of the clinical staging for nearly every tumor type. However, the conventional dogma of the metastatic cascade has largely ignored the relative contribution of the lymphatic system. We are interested in understanding how cancer survives within, and perhaps most importantly, how it leaves lymph nodes to subsequently colonize distant sites. Because the lymphatics drain into the circulatory system, discerning the relationship of cancer spreading via the lymphatic versus the direct hematogenous route is a complex problem. We are integrating autopsy datasets and developing new models to uncover previously unappreciated ways cancer spreads.
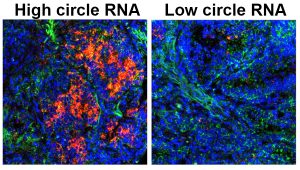
Role of non-coding RNAs in Metastasis:
Many metastatic traits require plastic processes that orchestrate “on/off” programs which enhance a cancer cells’ fitness to spread. We are studying how non-coding RNAs (such as miRNAs, circle RNAs and snoRNAs) regulate these events, and how these targets can be “actioned” as novel therapeutic strategies. Previous and ongoing work is leveraging integrated bioinformatic datasets, innovative metastasis models and therapeutic RNA interference strategies to tackle these problems (Harrison et al, Cancer Research, 2020; Pecot et al, Nature Communications, 2013). Our ultimate hope is that non-coding RNAs will create a new arsenal of cancer targets for more effective treatment strategies.
Role of Inflammatory Monocytes in Metastasis:
Traditionally, inflammatory monocytes have been regarded as inactive “precursors” to eventually become tumor-associated macrophages or dendritic cells. However, we recently found that inflammatory monocytes themselves can directly promote lung squamous metastases (Porrello et al, Nature Communications, 2018). Ongoing work is focused on uncovering new ways in which these cells can promote metastasis, and how they can be therapeutically targeted.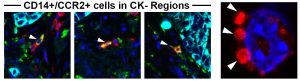
Role of Tumor Angiogenesis in Metastasis:
Although it is well known that cancers require blood vessels for their progression, most anti-angiogenic therapies have led to only a modest incremental improvement in survival. This has lead many groups to seek new ways to regulate tumor angiogenesis. Recently we found that Quaking, a critical driver of embryologic angiogenesis, gets re-activated in tumor endothelium to promote vessel formation (Azam et al, Oncogene, 2019). Ongoing work is studying the role of tumor endothelial Quaking on the surrounding tumor microenvironment as well as its potential as a novel anti-angiogenic target.
Development of Therapeutic RNA Interference to target Metastasis:
Although small molecule inhibitors and monoclonal antibodies have revolutionized oncology care, many cancer-relevant targets are regarded as “undruggable” using these approaches. Such targets include, but are not limited to, transcription factors, non-coding RNAs or difficult-to-target proteins (e.g. mutant KRAS). A common theme within nearly all of our projects is to study such pathways and utilize therapeutic RNA interference approaches to develop novel therapeutics. We are working hard to implement several state-of-the-art approaches using oligonucleotide chemistries, targeting modalities and delivery systems to help turn the RNAi dream into a reality for cancer patients (Pecot et al, Nature Reviews Cancer, 2011; Pecot et al, Nature Communications, 2013; Pecot et al, Molecular Cancer Therapeutics, 2014; Harrison et al, Frontiers Pharmacology, 2018).