Research
Genetics and Tumor Biology of Bladder Cancer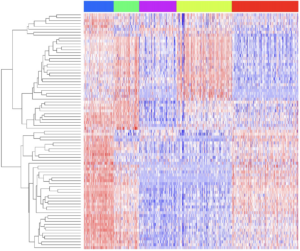
In 2021an estimated 83,730 adults (64,280 men and 19,450 women) in the United States will be diagnosed with bladder cancer. Our lab, contemporaneously with other groups around the country described the basal-like and luminal molecular subtypes of muscle-invasive bladder cancer [MIBC] (Damrauer JS, PNAS, 2014). As part of the TCGA Bladder Cancer Project we noted that a number of genes were mutated at a high frequency in MIBC. The lab is now focused on understanding how these genes contribute to the initiation, progression, and basal-luminal differentiation of bladder cancer using organoids, single-cell RNA sequencing (scRNAseq), and faithful preclinical mouse models.
Projects understanding the role of the NRF2 transcription factor, KDM6A, and FGFR3 are underway.
Improving Immunotherapy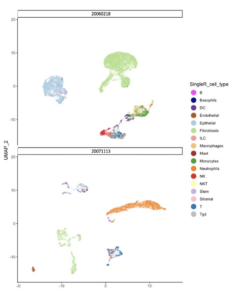
There are now numerous immune checkpoint antibodies (anti-PD1 and PD-L1) that are used to treat advanced bladder and kidney cancer in the clinic. While some patients have dramatic benefit from these immunotherapies, the majority of patients do not respond or have relatively short lived responses. One major goal of the lab is to “make immunotherapy better”.
With the Vincent Lab (UNC), we recently demonstrated that HDAC inhibition promotes immune-editing of tumor neoantigens through “neoantigen unlocking” and that this translates into long-term immunologic memory when combined with anti-PD1 (Truong AS, JCI, 2021).
Research projects identifying other therapeutic combinations that can synergize with immune checkpoint inhibition are underway.
Cancer Associated Fibroblasts (CAFs) and Immunotherapy Response
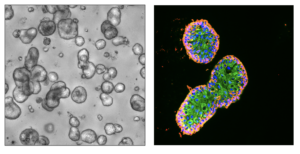 Cancer associated fibroblasts (CAFs) have been shown to play an important role in bladder cancer response to immunotherapy. Preliminary work from our lab shows that there is significant heterogeneity within CAFs. We are actively researching how these CAF subtypes affect the tumor biology of bladder cancer as well as therapies that can be used to specifically manipulate them.
Cancer associated fibroblasts (CAFs) have been shown to play an important role in bladder cancer response to immunotherapy. Preliminary work from our lab shows that there is significant heterogeneity within CAFs. We are actively researching how these CAF subtypes affect the tumor biology of bladder cancer as well as therapies that can be used to specifically manipulate them.
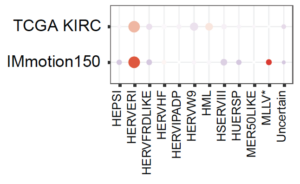
Non-canonical Tumor Associated Antigens in Renal Cell Carcinoma
Approximately 65,000 new cases of renal cell carcinoma (RCC) occur annually in the United States and its incidence is on the rise. RCC has historically been considered a relatively immunotherapy responsive disease, but perplexingly RCC has had a relatively low tumor mutational burden (TMB) and neoantigen burden. We hypothesized that non-canonical tumor associated antigens might serve as a nidus for adaptive immune responses. Our group recently demonstrated that inactivation of the tumor suppressor gene, PBRM1, cooperated with the hypoxia-inducible factor (HIFα) transcription factor to upregulate expression of endogenous retroviruses (ERVs) [Zhou M, Cancer Immunology Research, 2022]. These findings explain the clinical association between PBRM1 mutations and enhanced response to immune checkpoint blockade.
Current projects are examining the regulation and role of other non-canonical tumor associated antigens in immune checkpoint blockade benefit.