Our Research
The Chung lab harnesses the anti-tumor potential of our immune system via synthetic biology.
In our body, T cells – including CAR-Ts – differentiate into multiple cell states with distinct functions, migration patterns, and memory capacities. Although our understanding of individual T cell states has increased considerably over the past decade, a systems-level understanding of the T cell landscape is at its early stage. Moreover, we have limited toolkits for engineering these cell types.
By leveraging on our laboratory’s expertise in T cell differentiation and synthetic biology, we aim to enhance our own immune system’s natural abilities to fight cancer. More specifically, we are (1) generating a compendium of master regulator combinations of different T cell states and (2) creating advanced synthetic biology toolkits for artificial cell state differentiation. In the longer term, we will modulate the crosstalk between T cells and tumor microenvironment (TME) via designer oncolytic viruses.
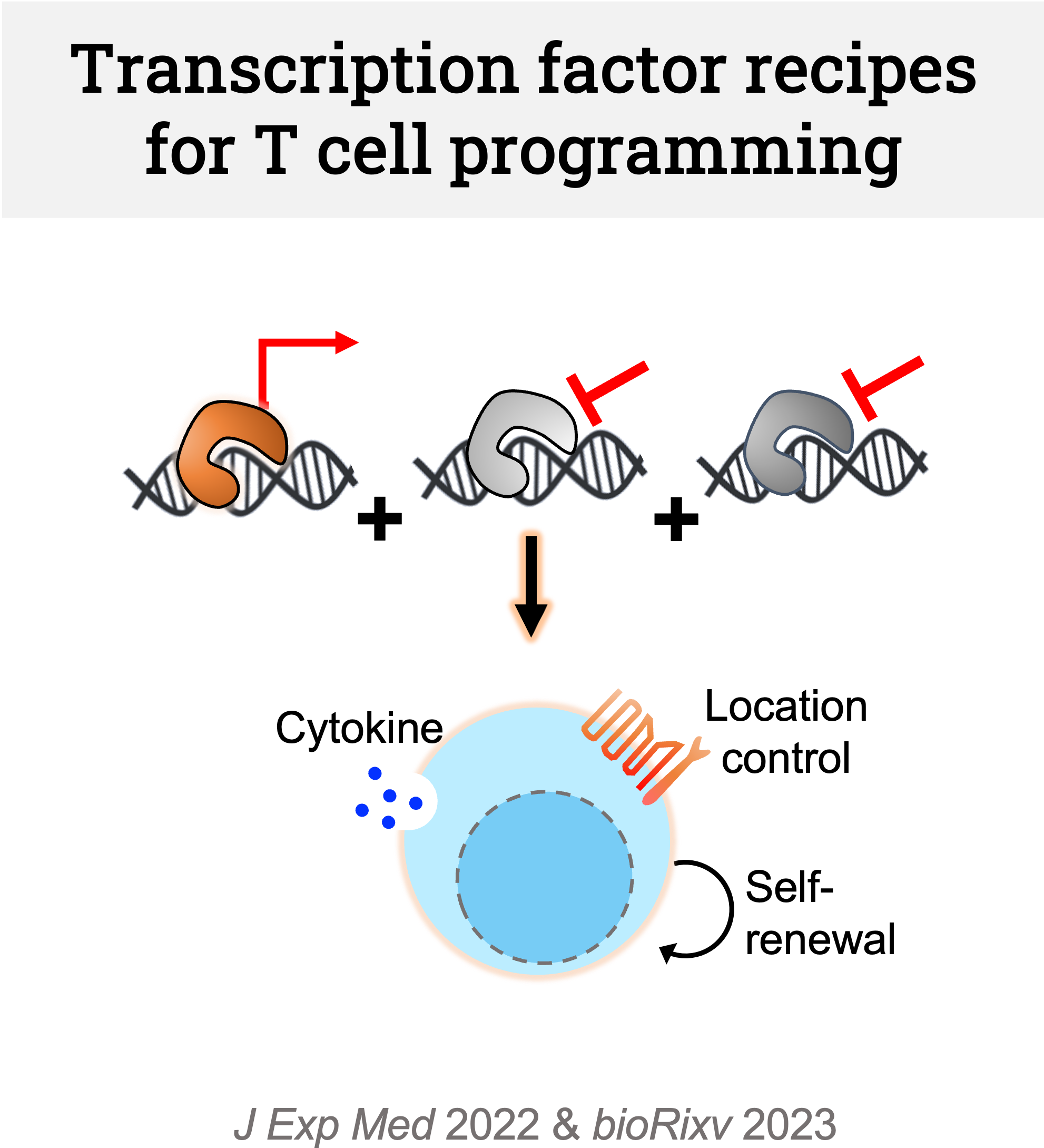
We are identifying transcription factor recipes to transform T cells into ideal therapeutic states. We combine (1) multiomics atlas-based transcription factor analysis platform and (2) in vivo single-cell CRISPR screening. We have discovered novel transcription factors whose perturbation synergizes with immune checkpoint blockade, enhancing tumor control. We are now screening for transcription factor combinations whose simultaneous perturbation allows effective cell state reprogramming (vs single knockout).
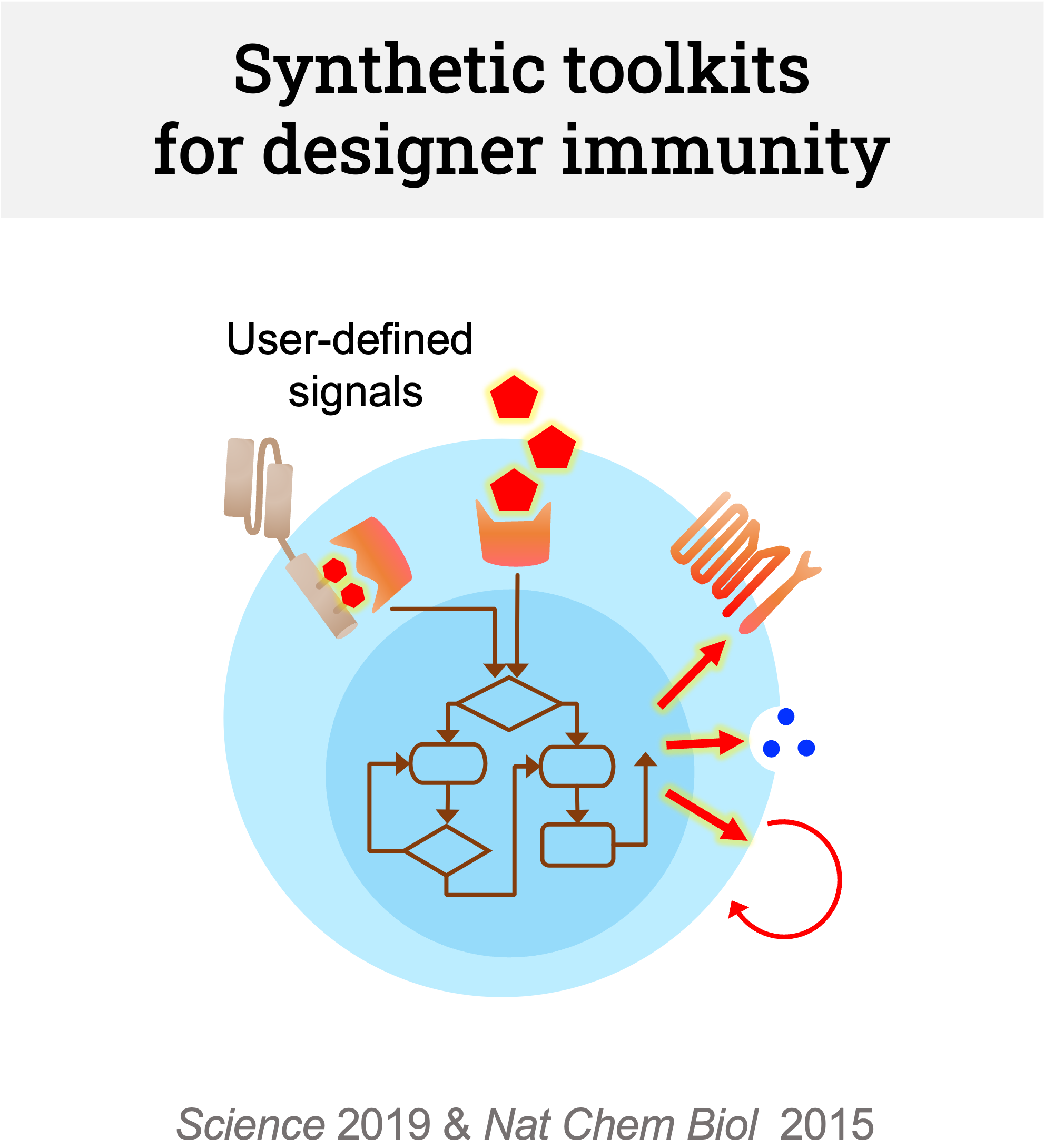
We are developing clinically applicable synthetic biology tools for context-specific cell state programming. Based on our previous works, we are currently working on two different platforms: (1) drug-inducible transcription factor circuits that allow temporal switching of cell states and (2) signal rewiring platforms for immune receptors such as chemokine, inhibitory, and nuclear receptors, which redirect user-defined unwanted signaling activation to execute therapeutic responses.
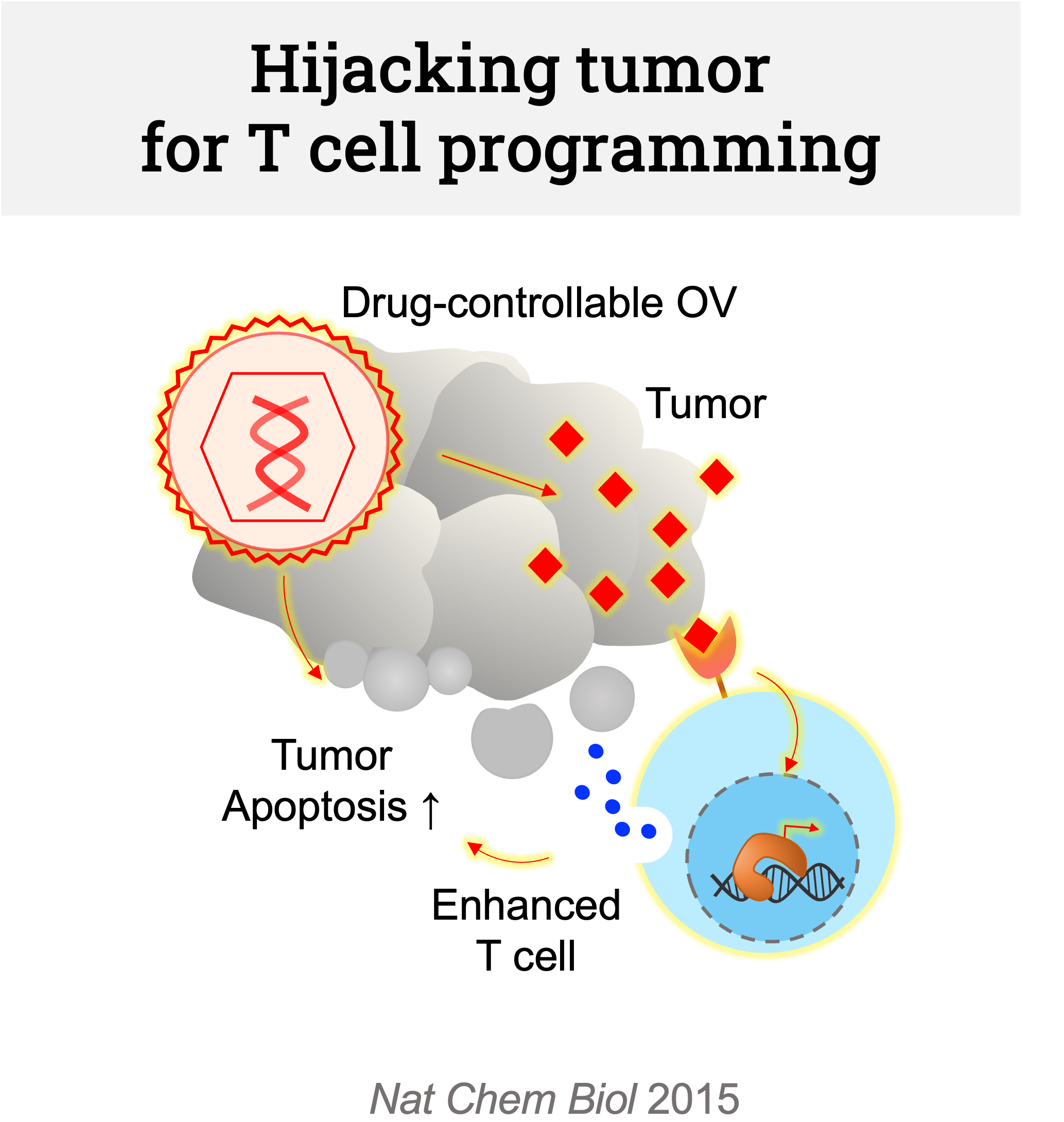
We are hijacking tumors’ translational system with oncolytic viruses (OV) to encode powerful – yet controllable – immune and TME modulators. In the past, we have developed the first drug-controllable, synthetic oncolytic RNA virus. Building upon this work, we now want to engineer the virus to facilitate synthetic crosstalk between tumor and T cell. This will enable long-lived memory T cells that can prevent future tumor recurrence.