Getting Started
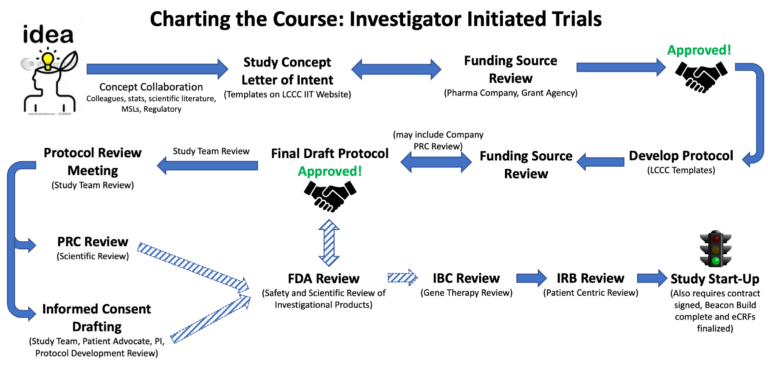
The process to move an Investigator Initiated Trial (IIT) from an idea to an accruing clinical trial involves many development and regulatory steps. The following graphic provides an overview of the process. Your clinical development team is here to help you through release of the final documents to your regulatory team so that they can submit to the Institutional Review Board (IRB) and other applicable committees such as the Institutional Biosafety Committee (IBC) for review.
Click to expand image
Note: FDA review is only applicable to some clinical trials (refer to our FDA page for details). IBC submission is only required for protocols using recombinant or synthetic nucleic acids (e.g., CAR-T cells, T-VEC, PANDA-VAC).
I have an idea for a Letter of Intent (LOI) or an approved concept. Now what?
The steps below give you an overview of the process that you need to take. Additionally, please refer to the investigator training “What an Investigator Needs to Know About Development of a Concept into an Enrolling Clinical Trial”. Overall, this process can take 6-12 months depending on the funding source review timelines and the engagement of the investigator.
1. Write an LOI and Put Together a BudgetResources: Funding Source Templates, LCCC LOI Templates
Who is there to help? Clinical Protocol Development Associates, IND Specialists, Biostatisticians
Tips:
- Involve your IND team early! You don’t want to develop a concept too far that will never get FDA approved.
- Clinical development can help you learn from UNC Lineberger’s combined experience! Multiple brains are better than one!
- Focus on getting a good budget! This is really your one opportunity.
Resources: LCCC Protocol Templates, Funding Source Template Language (SAE reporting, etc.), package inserts, and Investigator’s Brochures (as applicable).
Who is there to help? Clinical Protocol Development Associates, Biostatisticians
Tip: After your concept or LOI is approved and the funding source requests a protocol, talk with MSLs (or other company contacts) and clinical protocol development associates to get a good idea early of what the funding source’s review process will entail.
Who is there to help? Clinical Protocol Development Associates
Who is there to help? Clinical Protocol Development Associates, Biostatisticians, Regulatory Associate, In-House Monitor, Study Coordinator, Clinical Data Management Associate, IND Specialist (if applicable), Multicenter Project Manager (if applicable), Multicenter Regulatory Associate (if applicable), GMP Director (if applicable), anyone else you want!
Tips:
- Invite Co-Is!
- Listen to your team! It will lead to less confusion and amendments.
Resources: PRC Coversheets, PRC Charter
Who is there to help? Clinical Protocol Development Associates (PRC Coversheet, Submission & Responses), Biostatisticians (Stat sign-off & responses)
Tip: The PRC Charter contains the review criteria that will be used to “judge” your protocol!
Resources: LCCC Consent Form Templates
Who is there to help? Clinical Protocol Development Associates or IND Specialist, Patient Advocates
Tips:
- Must be at an 8th grade reading level! Microsoft Word has a tool to help determine this.
- Patient advocates help make sure your ICF is truly easy to read.
- FDA will want to see it!
Resources: LCCC IND Templates
Who is there to help? IND Specialist
Tip: FDA review takes 30 days, and they have a lot of last minute comments in the last week of the review—be prepared!
Resources: IBC application
Who is there to help? Regulatory Associate
Tip: Due the 15th of the month the month prior to the review — get this submission in early so it doesn’t delay your activation!
Who is there to help? Regulatory Associate
Tip: You can’t submit until you are FDA safe to proceed!
Who is there to help? Regulatory Associate, Study Coordinator, Financial Analyst, Activation Project Manager, Clinical Data Management Associate (eCRFs), Contract Analyst
Tip: To open a trial to accrual you need:
- An executed contract with a negotiated (if are receiving funding and/or drug from a pharma company)
- Beacon Build
- Investigational Drug Services Approval
- eCRFs
- IRB approval