Activation of the Multicenter Sites
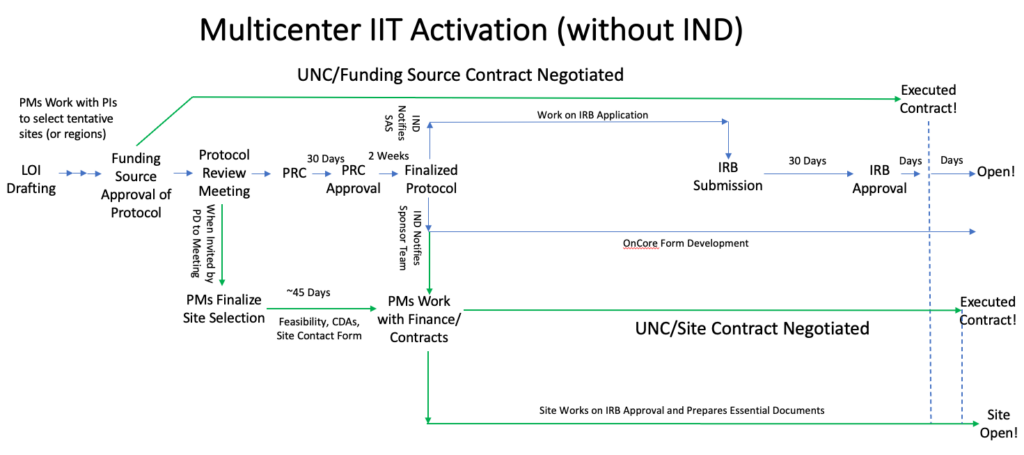
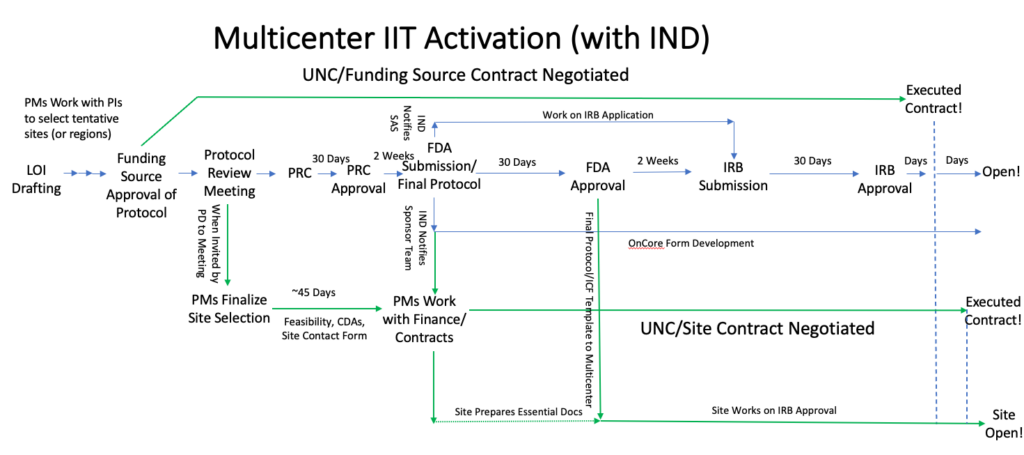
The timing of multicenter site activation differs between studies with and without INDs, but the overall processes are the same.
Click to expand image
Click to expand image
When can you start activating your multicenter sites?
Site Selection:
When your protocol is ready for a protocol review meeting!
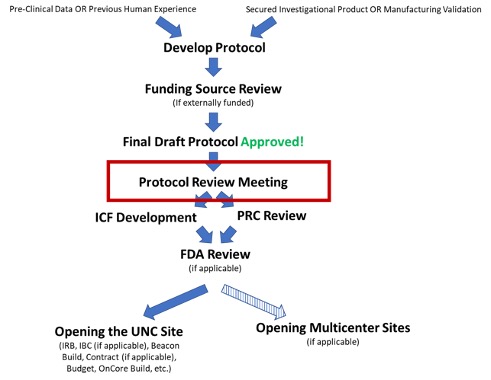
Feasibility, CDAs and Site Contact Forms:
When your protocol is ready for a protocol review meeting!
Opening the multicenter sites:
When the finalized protocol is released!
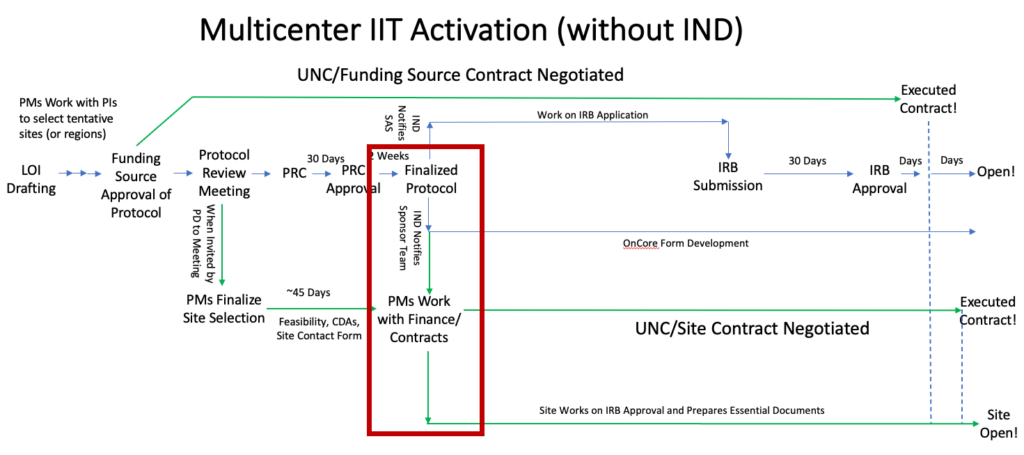
For studies without an IND, this occurs after the PRC comments are resolved:
Click to expand image
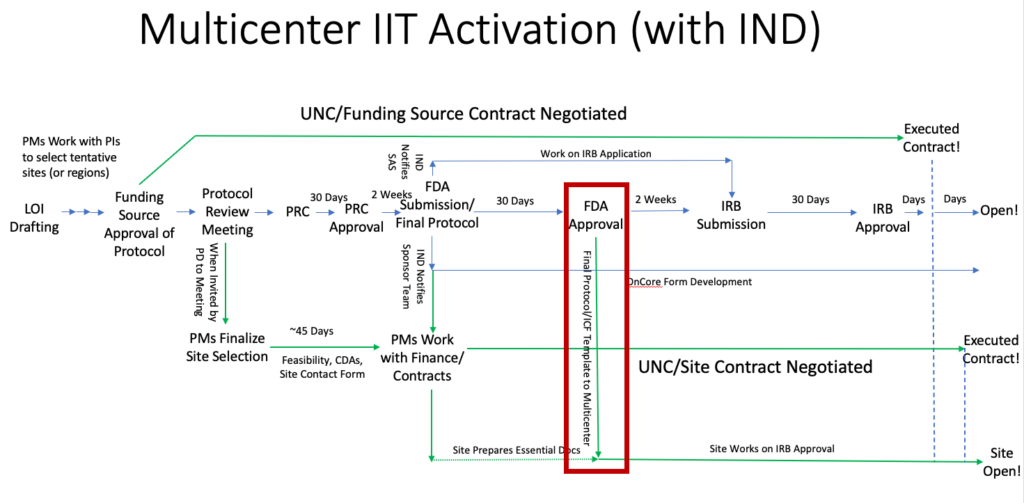
For studies with an IND, essential documents at the site start to be prepared based on the protocol submitted to the FDA, but full regulatory submissions start after FDA safe to proceed for the IND:
Click to expand image
What are the steps in multicenter site activation?
- Site selection
- Establish vendor for drug distribution if applicable.
- Completion of site feasibility form, contact information form and CDA execution
- Providing site with essential regulatory and protocol documents
- Concurrent processes at the site:
- Contract negotiation
- IRB approval
- Executed contract
- Site initiation meeting (SIM)
- Site open to accrual!
Who is there to help me with multicenter activation?
Your multicenter project manager and multicenter regulatory associate!
Multicenter Project Managers:
Multicenter project managers help with the following during activation:
- Works with the PI to identify multicenter sites providing information about LCCC’s previous experience working with those partners
- Works with vendors, purchasing office, contracts and budget team to establish vendors for drug distribution as applicable for the study.
- Works to determine site feasibility and collect site contacts
- Submits CDAs to our contracts team so you can send the clinical protocol to the sites
- Project manages all activation from the other teams:
- Budget negotiation
- Contract negotiation
- Completion of essential regulatory documents
- Acquiring local regulatory approvals
- Ensuring site approved by FDA (if applicable)
- Lead the Site Initiation Meeting (SIM)
Multicenter Regulatory Associates:
Multicenter regulatory associates help with the following during activation:
- Acquiring completed essential regulatory documents from the sites
- Providing protocol and essential documents to the sites
- Ensuring site IRB approval
- Reviewing/approving site consent forms